Most Common Immediate Complication Following Surgical Repair Of An Abdominal Aortic Aneurysm?
Review Article
Complications of endovascular aneurysm repair of the thoracic and intestinal aorta: evaluation and management
Introduction
Endovascular repair of the thoracic and abdominal aorta is an important advance in the treatment of aortic aneurysms and other aortic pathologies. Since the U.S. Food and Drug Assistants (FDA) canonical the use of endograft devices, in that location has been a 600 per centum increment in the annual number of endovascular aneurysm (or aortic) repair (EVAR) procedures performed (1). Endovascular abdominal aortic aneurysm (AAA) repair and thoracic endovascular aneurysm repair (TEVAR) currently business relationship for nearly l% of all aortic aneurysm repairs that are performed in the U.S. (ane). In recent years, at that place has been an overall subtract in the incidence of ruptured aneurysms, likely due a combination of improved AAA screening, and increased rates of constituent endovascular repairs in patients who would not otherwise be surgical candidates (1). While these techniques were initially used for the treatment of patients who were accounted loftier-gamble surgical candidates, emerging information in recent years proving their prophylactic profile have made EVAR the preferred treatment techniques for many patients with aortic aneurysms due to the decreased perioperative morbidity and the comparable to improved outcomes of these procedures relative to open surgical repair (two-9).
EVAR involves the placement of a prosthetic endograft within the thoracic or abdominal aorta at the site of an aneurysm or other pathologic process that threatens the integrity of the aorta. The diverse endograft components are typically compressed within a delivery sheath and are introduced into the vascular organization through the lumen of an access vessel, to be subsequently deployed at the site of the aneurysm. In one case deployed at the target site of handling, the endograft self-expands to contact the aortic wall thereby excluding the weakened aortic wall or aneurysm sac from the pathologic increased menstruation and pressure that might otherwise lead to aortic/aneurysm rupture. Among the most important determinants for the success of an endovascular repair are the anatomic suitability of the patient's vasculature for device placement, and the choice of an endograft that is of advisable size and configuration for the patient'south anatomy and aortic morphology. The device must provide acceptable seals or fixation proximally and distally at the endograft landing zones in order to successfully exclude the aneurysm sac. To be a suitable candidate for EVAR, certain full general anatomic criteria must be fulfilled including an aortic aneurysm proximal neck size that measures xviii–32 mm in bore and is greater than ten mm in length, a neck angulation that is typically less than 45–60 degrees (depending on the device used), a common iliac artery bore betwixt 8–22 mm and an external iliac diameter greater than 7 mm (ten). If the planned positioning of the endograft is expected to encompass important aortic side co-operative vessels, debranching procedures may be needed prior to graft placement or fenestrated endografts may be required.
AAA repair is indicated in patients with symptomatic aneurysms, in those who have an aneurysm diameter greater than 5.v cm, or in those whose aneurysm has expanded by more than 0.v cm in a 6-month interval (2). Similarly, repair is indicated for thoracic aortic aneurysms in symptomatic patients, patients with an aortic size index equal to or greater than 2.75 cm/m2, patients with aortic diameters of vi to 7 cm, patients with genetically-mediated conditions that are associated with aortic pathology or patients with aneurysm bore expansion of greater than 10 mm per year (11-thirteen). In patients whose anatomic criteria are suitable, EVAR is typically the preferred means of treatment. Absolute contraindications to EVAR include various unfavorable anatomic features such as excessive aortic tortuosity and angulation, a hostile proximal neck with circumferential calcification, excessive mural thrombus or an extremely conical configuration, and extremely small-caliber access vessels. There are also sure relative contraindications such every bit the inability or unwillingness to comply with post-procedural surveillance imaging.
EVAR of the intestinal aorta conveys a number of advantages when compared to open aneurysm repair. Available information show perioperative survival benefit as compared to open up surgery. In a systematic review of 1,532 patients, endovascular repair was associated with a significantly lower thirty-24-hour interval mortality (1.vi%) than open surgery (4.viii%) (14). The survival advantage conveyed by endovascular repair is even greater in high-risk surgical candidates where the 30-day post-process mortality charge per unit was found to be 4.7% compared to 19.2% in those who underwent open repair (fifteen). To our knowledge, no randomized studies are available comparing open and endovascular repair in the thoracic aorta. However, observational studies suggest equivalent or meliorate overall outcomes (16). EVAR of the abdominal aorta is likewise associated with a significant reduction in perioperative morbidity when compared to open up surgery, with decreased blood loss, elimination of the need for cross-clamping the aorta intraprocedurally and shorter recovery periods (1,17-20). Specific to thoracic aneurysm repair, TEVAR provides the reward of avoidance of sternotomy and thoracotomy, both of which carry high patient morbidity (20).
While EVAR is associated with improved short-term survival in patients with aortic aneurysms, information technology is of import to annotation that available information do non testify long-term improvement in survival benefit when compared to open repair. In a review of 22,830 matched Medicare patients who underwent endovascular and open repair, lower perioperative mortality was again demonstrated (21). However, the overall mortality was similar between the two groups at 3 to 4 years post-procedure (21). Considering endograft imaging surveillance is mandatory for the residuum of a patient's life later on EVAR, the gamble of the long-term radiations exposure associated with imaging makes the use of this technique somewhat controversial in young patients who are otherwise expert surgical candidates, given the equivalent long-term survival outcomes of the 2 techniques. The decision to pursue endovascular or open repair should be personalized to each patient and should be based on the patient's age, surgical risk and vascular anatomy.
With EVAR, the preferred intervention for the bulk of patients with aortic aneurysms, an increasing number of complications are beingness reported as a result of the marked increase in the number of these procedures that are being performed (22). Emerging data show that endograft-related complications are relatively common. Post-obit EVAR for AAA, the rate of complications has been reported to range betwixt 16% and thirty% with secondary interventions needed in up to nineteen% of patients (23-28). For TEVAR, belatedly complications have been shown to occur in up to 38% of patients with secondary intervention required in approximately 24% of cases (9,29-32). In this commodity, nosotros summarize the current surveillance recommendations for detecting and evaluating complications post-obit EVAR of the thoracic and abdominal aorta. We also provide an overview of commonly reported complications and discuss the secondary interventions typically performed for treatment.
Endograft surveillance and evaluation
Electric current surveillance recommendations
Lifelong post process imaging surveillance is currently recommended in all patients post-obit endovascular repair of the thoracic and abdominal aorta so as to evaluate the long-term performance of the endoprosthesis. Imaging is essential for assessment of the integrity of the endograft and for confirmation of the stability of or a decrease in the size of the excluded aneurysm sac. If a post-procedural complication or abnormality is detected by clinical or imaging surveillance, the latter can as well exist used to farther evaluate and narrate the aberrant finding; commonly occurring postal service-procedural problems include endoleaks, endograft migration or collapse, limb kinking and/or stenosis and endograft infection. Imaging techniques that are used for surveillance include conventional radiography, computed tomography (CT), ultrasonography, nuclear imaging, magnetic resonance angiography (MRA) and conventional angiography, with CT considered every bit the gilt standard modality. These techniques are summarized in Table i. Current guidelines for surveillance imaging postal service-endovascular repair recommend imaging at 30 days, 6 and 12 months following the procedure and yearly thereafter, if no complications are detected (11,33).
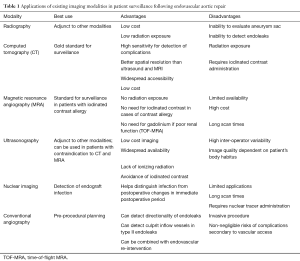
Table 1 Applications of existing imaging modalities in patient surveillance following endovascular aortic repair
Full tableConventional radiography
Conventional radiography can provide an overview of graft positioning and integrity and conveys the advantage of low surveillance cost and low radiation exposure (34). Anteroposterior (AP) radiographs can be helpful in detecting endograft migration and separation of modular endograft components (35). Supplemental oblique views tin be used to detect wire fractures (35). However, conventional radiography is rarely used lone for post-procedural surveillance simply may instead exist used as a complement to other imaging modalities. At that place are multiple disadvantages of using conventional radiography for surveillance, such as the inability to evaluate the size of the residual aneurysm sac or to detect soft tissue and flow-related complications such as endoleaks and graft infections, many operators and institutions no longer routinely use this imaging technique for endograft evaluation.
CT and CT angiography (CTA)
CT is considered the golden standard technique for surveillance imaging in patients who have undergone EVAR. Typical CT imaging protocols include a non-contrast phase, an arterial imaging phase and a delayed imaging phase at 120–300 seconds. Not-contrast imaging is necessary and so every bit to differentiate high density material such as calcification that may be present in the aneurysm sac from abnormalities such as endoleaks that may exist seen on subsequent later phase imaging. Arterial and delayed-phase imaging are used to assess endograft integrity, to notice and characterize endoleaks and to assess for the presence of other abnormalities such every bit limb apoplexy or endograft infection. The bore or volume of the residual aneurysm sac should be measured on each surveillance scan in order to ensure stability or to demonstrate a decrease in the size of the excluded sac. We typically measure the largest diameter of the aneurysm sac using a double-oblique short-axis orientation, so every bit to improve measurement accurateness and increase inter-reader reproducibility. Many operators abet calculation of the residue aneurysm sac book equally the most accurate measurement, if appropriate post-processing software is available.
CTA provides 92% sensitivity for the detection of endoleaks and offers better spatial resolution for the cess of the endograft relative to ultrasonography and MRA (36,37). In addition, CT conveys the advantage of widespread accessibility and relatively low cost when compared to other imaging modalities. Despite these advantages, significant concerns remain about the cumulative radiations exposure and the demand for the repetitive administration of iodinated dissimilarity (34,38). Cumulative radiation exposure is of particular concern in younger patients undergoing yearly surveillance CT scans. Similarly, administration of iodinated dissimilarity is problematic in patients who are at take a chance for contrast-induced nephropathy. Recent advances in dual-source dual-energy CT and other paradigm reconstruction approaches are promising for radiations dose reduction (39,xl). Reduced dissimilarity-dose techniques are also being actively explored so as to reduce the risk of contrast-induced nephropathy (41).
MRA
MRA is considered an culling to CTA for post-EVAR surveillance imaging (42). Typical imaging protocols include an centric T1-weighted gradient echo sequence, a unmarried-shot fast spin echo sequence and pre- and post-contrast sequences. Not-contrast time-of-flight MRA (TOF-MRA) imaging can also be performed and is especially useful in patients with poor renal function or those who have a contraindication to gadolinium use. Unlike CT, TOF-MRA besides allows the detection of the directionality of blood menstruum. Recent data suggest that MRA is superior for the imaging of nitinol endografts as compared to CT (43). Otherwise, gadolinium-enhanced MRA is equivalent to CTA in sensitivity for the detection of endoleaks. When TOF-MRA imaging is used alone in patients with a low estimated glomerular filtration charge per unit (eGFR), its sensitivity for endoleak detection can be as depression as 54% (44). However, when TOF is used in conjunction with gadolinium-enhanced MRA, information technology has 97% concordance with angiography for the detection of endoleaks (45).
MRA conveys several advantages when compared to CTA including the use of non-ionizing radiations for imaging and the avoidance of the assistants of iodinated IV contrast. MRA is specially useful in patients who take an iodinated contrast allergy or who have other contraindications to receiving these contrast media. Potential drawbacks to MRA use include the more limited availability of MR as compared to CT, the higher imaging costs, longer scan conquering times, use in claustrophobic patients and the inability to clear all patients for imaging by magnetic resonance. In patients undergoing dissimilarity-enhanced magnetic resonance imaging (MRI), emerging bear witness likewise suggests that there is gadolinium deposition and aggregating in the central nervous arrangement fifty-fifty in patients with normal renal function (46,47). Although the clinical significance of gadolinium deposition in the brain remains unclear, care should be taken when using MRI for patient surveillance until further data is available about the long-term safe of gadolinium-based agents.
Ultrasonography
Ultrasound can be quite useful for the surveillance of a patient post-obit EVAR. A typical post-procedure ultrasound protocol includes B-mode imaging of the abdominal aorta, iliac arteries and femoral arteries in transverse and longitudinal orientations in order to assess the endograft, the landing zones and the size of the balance aneurysm sac. The examination should also include the utilize of color and power Doppler and so as to confirm endograft patency and to assess for flow directionality and the presence or absenteeism of endoleaks. Emerging data advise that contrast-enhanced ultrasound (CEUS) with not-targeted microbubbles can exist used to enhance the sensitivity of ultrasound in endoleak detection (48). A recent systematic review likewise suggested that CEUS has high sensitivity for detecting endoleaks and can be introduced every bit a routine diagnostic modality to exist followed past CTA only when the ultrasound is positive to further characterize an endoleak (49). Ultrasound is reported to take a specificity of 93–94% and a sensitivity of 70–82% for the detection of endoleaks (49-51).
Ultrasound imaging for surveillance following EVAR offers the advantage of low-toll imaging, widespread availability, a lack of ionizing radiation and the avoidance of iodinated contrast utilise. Nonetheless, ultrasound suffers from high inter-operator variability and the quality of the collected images is highly dependent on the patient'southward body habitus. Evaluation of endograft integrity and positioning is too limited with ultrasound. Accordingly, ultrasound remains an adjunctive technique in surveillance and is rarely used as the sole surveillance tool unless the patient has contraindications to both CT and MRA.
Nuclear imaging
Nuclear imaging techniques have been found to generally be useful for the detection and label of an endograft infection, with labeled white blood cell (WBC) imaging, gallium scanning and FDG-PET imaging all having demonstrated roles (52,53). The sensitivity of these techniques for the detection of endograft infection has been reported to range between 60% and 100% (52,54). Nuclear imaging is specially useful in the immediate post-operative period when endograft infection is suspected. It has been shown that nuclear imaging during that period is more sensitive than CT for the detection of graft infection (52,55).
The use of 99mTc-labeled reddish blood cells and technetium-99m sulfur colloid has been proposed for the detection of endoleaks in post-endovascular repair surveillance imaging. Nevertheless, bachelor data show that these techniques exhibit significantly lower sensitivity when compared to CT (56,57).
Conventional angiography
CTA and MRA are more sensitive than digital subtraction angiography (DSA) for the detection of complications following EVAR (34,36). DSA is currently used for pre-procedural planning or intraprocedural guidance prior to or during secondary interventions. Post-procedural DSA as well allows one to featherbed immediate follow-upwardly post-procedural imaging in patients who have clinically apparent post-EVAR complications. DSA is especially useful for the detection of the directionality of an endoleak and for identifying the culprit inflow vessel in type Two endoleaks. DSA, however, is an invasive process and carries not-negligible risks including admission site complications such every bit hematoma or pseudoaneurysm formation, and other agin events like arterial dissection or thrombosis, retroperitoneal hemorrhage, and vessel rupture.
Endovascular repair complications and their management
Device-related complications
Endoleak
Endoleaks are the near commonly occurring complication following EVAR. The nearly common complications are summarized in Table 2. Endoleaks represent persistent blood flow perfusing the residual aneurysm sac thus indicating failure to completely exclude the aneurysm. There are five types of endoleaks that take been extensively described (58-61). Type I endoleaks occur because of an incompetent seal at the proximal (type IA) or distal (type IB) endograft zipper site. Type Ii endoleaks are characterized by persistent flow into and out of the residual aneurysm sac via patent aortic side co-operative vessels such as the inferior mesenteric artery, lumbar arteries, accessory renal arteries or the left subclavian avenue. Type III endoleaks are caused by structural failure of the endograft itself. Examples include tears in the endograft fabric and separation or dehiscence of modular graft components. Blazon IV endoleaks are caused past graft porosity, while type 5 endoleaks are characterized past connected remainder aneurysm sac expansion despite the lack of whatsoever evidence of an endoleak by imaging, which is a phenomenon that is known every bit endotension. The near unremarkably occurring types of endoleaks following both thoracic and abdominal EVAR are types I and Ii. Endoleaks are more than commonly seen post-obit endovascular repair of the intestinal aorta and occur in 15–30% of patients in the showtime 30 days afterward the procedure (34,62). They are seen less commonly with TEVAR, occurring in 4–15% of the cases (nine,thirty,63). When present, endoleaks carry an increased hazard for connected aneurysm expansion and eventual rupture.
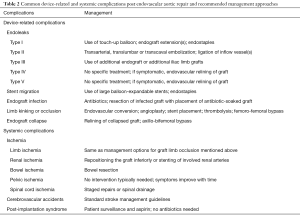
Table 2 Mutual device-related and systemic complications post endovascular aortic repair and recommended management approaches
Full tabular arrayBecause endoleaks are the most commonly occurring complication post-obit EVAR, secondary interventions for their treatment, when necessary, represent the most frequently performed post-repair process. Techniques for treating type I endoleaks are aimed at securing the involved proximal or distal endograft attachment site or seal zone (Figures 1,2). Potential techniques include using a large quotient compliant balloon to more optimally distend the endograft at the attachment site or to extend the endograft proximally or distally with an endograft aortic extension cuff or limb (64). Other operators advocate using a high radial-force stent at the proximal zipper site to more than securely seal the graft, while others take favored peri-graft embolization using a liquid embolic amanuensis such as n-butyl cyanoacrylate (65,66). More than recent techniques include the use of endostaples to secure the position of the primary aortic endograft or of an aortic extension gage to the native aorta (67,68). The virtually unremarkably used strategy for treatment of a proximal type I endoleak involves placement of an aortic cuff extension.
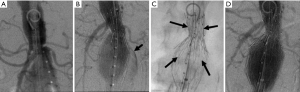
Figure ane Handling of a type IA endoleak. Intraprocedural DSA images testify (A) a brusque and conical proximal neck in a patient undergoing EVAR. This is a known predisposing cistron for a type IA endoleak; (B) post-obit deployment of the endograft, contrast can be seen coursing outside of the confines of the device (arrow) filling the aneurysm sac, a finding that is typical of a blazon I endoleak; (C) in order to maximally distend the endograft device at the proximal attachment site, a loftier radial force balloon-mounted bare-metal Palmaz® stent (arrows) was deployed; (D) later placement of the additional stent, the endoleak has been eliminated, with just the endograft filling. DSA, digital subtraction angiography; EVAR, endovascular aneurysm (or aortic) repair.
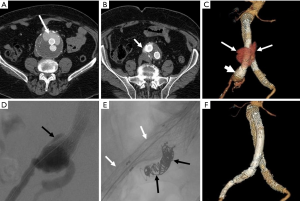
Figure 2 Treatment of a type IB endoleak. Axial contrast-enhanced mail service-EVAR surveillance CT images show (A) a large collection of contrast (arrow) anteriorly within the residual AAA sac external to the endograft limbs that (B) courses inferiorly (arrow) adjacent to the right iliac limb of the graft; (C) 3D image once more shows the endoleak (arrows) and also demonstrates that the distal aspect of the right iliac limb is flared (short arrow); (D) intraoperative DSA shows dissimilarity tracking exterior of the flared end of the right iliac limb (blackness arrow), a finding that is typical of a type IB endoleak; (E) intravascular coils (blackness arrows) were placed in the right internal iliac avenue so as to preclude retrograde perfusion of the expanse of endoleak and an additional modular graft limb (white arrows) was extended into the external iliac artery to attain a satisfactory distal seal; (F) 3D epitome following endoleak repair shows the last configuration of the right iliac limb every bit well as resolution of the endoleak. EVAR, endovascular aneurysm (or aortic) repair; AAA, abdominal aortic aneurysm; DSA, digital subtraction angiography; CT, computed tomography.
Secondary interventions for type II endoleaks almost always involve some type of embolization (Figures 3,4). Embolic occlusion of the patent aortic side branches that go along to perfuse the residual aneurysm sac or embolization of the nidus inside the residue sac are the commonly employed techniques, with the latter favored. Routes of admission for performing type 2 endoleak embolization include transarterial (69), percutaneous translumbar aortic (70), and transcaval approaches (71). Various embolic agents have been used including intravascular coils, liquid embolic agents such as due north-butyl cyanoacrylate, thrombin and ethylene vinyl booze copolymer (Onyx®, Covidien-Medtronic, Minneapolis, MN, United states) (72). Other described techniques include surgical ligation of the culprit arrival vessel (73,74). These embolization procedures conduct a risk for the development of ischemic colitis when the inferior mesenteric artery (IMA) is involved.
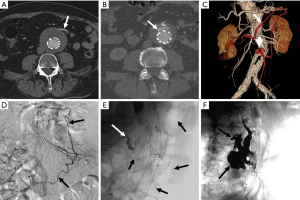
Figure 3 Treatment of a type 2 endoleak. Axial dissimilarity-enhanced post-EVAR surveillance CT images show (A) a contrast-filled patent inferior mesenteric artery (IMA) (arrow) emanating from the residual AAA sac; (B) a contrast collection inductive (arrow) to the endograft fills via retrograde flow within the IMA, which is typical of a type Ii endoleak; (C) a 3D image shows that the heart colic artery (white arrow), arising from the superior mesenteric artery (SMA) fills the left colic artery of the IMA (black arrow) in retrograde fashion, thereby perfusing the endoleak nidus (brusk arrow). This course is highlighted in red; (D) intraoperative DSA shows the same vascular arcade (arrows) that fills the type Ii endoleak nidus; (E) a microcatheter (black arrows) has been passed through this SMA to IMA route and contrast has been injected opacifying the nidus (white arrow); (F) the endoleak has been embolized using Onyx®, with the large cast (black arrows) evident in the AAA sac. EVAR, endovascular aneurysm (or aortic) repair; AAA, intestinal aortic aneurysm; CT, computed tomography.
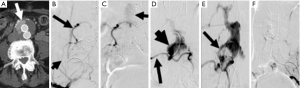
Effigy iv Translumbar treatment of a type Ii endoleak. Axial contrast-enhanced mail-EVAR surveillance CT image shows (A) a contrast (pointer) anteriorly within the residue AAA sac external to the endograft limbs; (B) intraoperative right internal iliac DSA shows filling of the iliolumbar avenue (short arrow) with retrograde perfusion of a lower lumbar artery (arrow); (C) a more than delayed prototype shows faint opacification of the endoleak nidus (arrow) within the aneurysm sac; (D) via percutaneous translumbar admission, a catheter (pointer) has been introduced directly into the residual AAA sac and dissimilarity has been injected, opacifying the nidus (short arrow) and boosted lumbar arteries; (Eastward) a combination of intravascular coils and liquid thrombin were used to embolize the nidus. A filling defect (arrow) in the base of the nidus is seen where the initial embolic agents have been introduced; (F) final image after translumbar embolization shows elimination of the type II endoleak. DSA, digital subtraction angiography; EVAR, endovascular aneurysm (or aortic) repair; AAA, abdominal aortic aneurysm; CT, computed tomography.
Management of type Three endoleaks unremarkably requires placement of additional modular endograft components to seal and re-plant the integrity of the affected portion(s) of the endograft (64) (Figures 5,half dozen). No specific treatment is recommended for blazon Iv and blazon V endoleaks. Notwithstanding, if treatment becomes necessary considering of continued expansion of the residual aneurysm sac, endovascular re-lining of the original endograft or open up surgical conversion may be necessary.
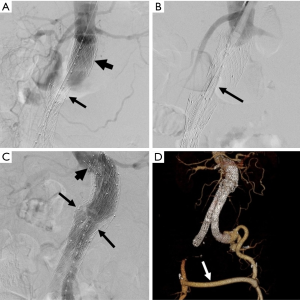
Effigy 5 Type 3 endoleak repair. (A) Intraoperative DSA shows consummate separation of components of a modular endograft, with the proximal aortic cuff (short arrow) remaining in the original position just with detachment of the endograft trunk (arrow), with an intervening segment in which there is no endograft, so that the AAA sac is no longer excluded; (B) a new endograft component (arrow) has been introduced and will exist used to bridge the separated endograft components; (C) after placement of the new component the integrity is restored (arrows). The new component extends proximally (brusque pointer) higher up the original aortic cuff; (D) the type III repair required extension of the new component via simply one limb of the bifurcated body of the original endograft. Thus, in order to perfuse the contralateral lower extremity, placement of a cross femoral bypass graft (arrow) was necessary, every bit seen in this 3D CT image. DSA, digital subtraction angiography; AAA, abdominal aortic aneurysm; CT, computed tomography.
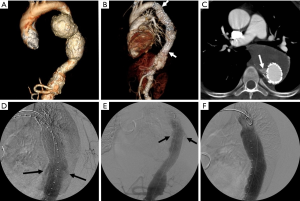
Figure six Endoleak following TEVAR. (A) 3D CTA paradigm of the thoracoabdominal aorta prior to TEVAR shows a large bilobed descending thoracic aortic aneurysm; (B) the aneurysm was treated with placement of a thoracic endograft (arrows); (C) axial image from a surveillance CT shows a contrast drove (arrow) adjacent to the endograft, c/w an endoleak; (D) intraoperative DSA shows that the endoleak (arrows) is side by side to overlapping modular components of the TEVAR endograft, indicating that this is a type Iii endoleak; (E) an additional endograft component was placed bridging the surface area from which the endoleak originates: prior to balloon amplification of the new component the endoleak is nonetheless axiomatic (arrows); (F) after distending the newly placed device component with a compliant balloon, the endoleak is eliminated. CTA, CT angiography; TEVAR, thoracic endovascular aneurysm repair; DSA, digital subtraction angiography; CT, computed tomography.
Endograft migration
Device migration is a mutual complication that requires secondary intervention following EVAR (75). It is defined equally deportation of the endograft by more v–ten mm from its original position (Effigy vii). It is often due to progressive dilatation of the aneurysm neck but can also exist related to aortic tortuosity, aortic wall degeneration after endograft placement or may exist secondary to graft over- or under-sizing. Device migration is associated with endoleaks, aneurysm sac expansion and possible rupture. Device migration has been reported to occur post-obit 1.0–2.8% of TEVAR procedures and 1–x% of endovascular repair of the abdominal aorta at 1 year mail service-intervention (vi,9,30,76). In cases of aortic endograft migration, treatment is very similar to management of a type I endoleak. Endovascular treatment options include the employ of aortic extension cuffs or placement of large airship-expandable stents to augment the fixation of the endograft to the native aortic wall and thus extend the fixation zone. Some other selection is that of using endostaples to secure the graft to the aortic wall (77,78).
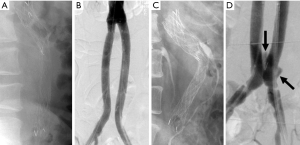
Effigy seven Endograft migration. (A) Lateral radiograph 1 month later on EVAR shows the expected configuration of the endograft mail-placement; (B) the intraoperative DSA at the fourth dimension of endograft placement shows a typical endograft configuration; (C) a lateral radiograph obtained 2 years subsequently EVAR shows that the endograft limbs are now bowed anteriorly. This modify in the endograft configuration occurred every bit a issue of remodeling of the residual aneurysm sac; (D) the distal ends of the iliac limbs have now migrated cephalad, resulting in blazon IB endoleak (arrows). EVAR, endovascular aneurysm (or aortic) repair; DSA, digital subtraction angiography.
Endograft infection
Endograft infection has been reported to occur in 0.iv–three.0% of cases following EVAR of the abdominal aorta (lx,61,79,80). It is associated with high mortality rates that range from 25% to 50% and that are usually secondary to septic shock (sixty,eighty,81). Endograft infection may be caused past intraprocedural contamination, in which instance the infection occurs early afterward the procedure. If the infection occurs at a afterwards fourth dimension subsequently repair, information technology may be the result of a remote site of infection leading to colonization of the endograft. Rarely, endograft infection may lead to aortoenteric fistula formation (80). Patients typically present with fever, leukocytosis and back pain. Endograft infections may be managed conservatively with antibiotics or may be treated aggressively with endograft explantation and placement of an antibody-coated graft (37,82,83). The clinical approach is highly dependent on the clinical scenario and the patient'due south comorbidities.
Limb kinking or occlusion
Kinking and/or occlusion of endograft limbs have been reported in 2–4% of patients following EVAR of the abdominal aorta (84,85) (Figure viii). Causes for these complications include progressive decrease of the size of the residue aneurysm sac over time, excessive aortic neck angulation and a narrow bore to the distal aortic cervix (86). Limb kinking tin atomic number 82 to type I and/or type III endoleaks as well as to endograft migration. It can too consequence in endograft limb thrombosis and occlusion which may in turn cause acute lower extremity ischemia. A number of treatment options are available for the treatment of limb kinking, stenosis or occlusion. Astringent limb kinking can be treated by placement of reinforcing stents or additional endograft limbs within the original graft. Percutaneous angioplasty can exist performed with or without additional endograft placement to treat limb stenosis or apoplexy. Additionally, occluded endografts may sometimes be treated with thrombolysis or thrombectomy and new limb placement. With thrombectomy, care has to be taken to avoid distal embolization into outflow runoff vessels. Other options include a cross-femoral surgical bypass, which may often be the preferred procedure. Timely direction of this complexity is especially of import then as to subtract the likelihood of distal limb ischemia and to improve patient outcomes.
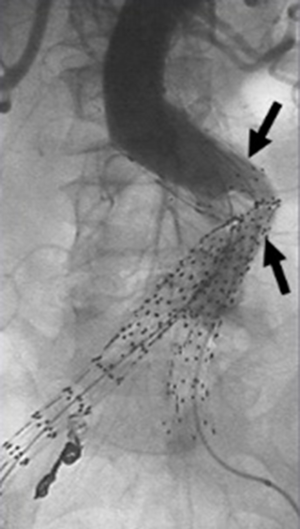
Figure 8 Kinked endograft limbs. DSA shows kinked endograft limbs (arrows), a miracle that may occur in association with remodeling of the AAA sac following EVAR, and may pb to limb apoplexy and thrombosis. DSA, digital subtraction angiography; AAA, intestinal aortic aneurysm; EVAR, endovascular aneurysm (or aortic) repair.
Endograft collapse
Device infolding or collapse has been reported following TEVAR. Information technology is idea to be related to a minor proximal aortic curvature or may be associated with oversizing of the endograft relative to the native aorta (87,88). A bird-beak configuration of the endograft is significantly correlated with the run a risk of type IA endoleak formation, and is a potential risk gene for proximal endograft plummet or infolding (Figure 9). Endograft collapse is virtually commonly seen after endovascular repair of traumatic aortic injuries (89). Endograft collapse typically occurs within the first 30 days afterward the process, with a median time to collapse of xv days, as reported in a review of 60 cases of endograft collapse post-obit TEVAR (87). A high level of suspicion is needed for the diagnosis of endograft collapse in the first 30 days after the process. Patients typically present with symptoms of acute aortic occlusion.
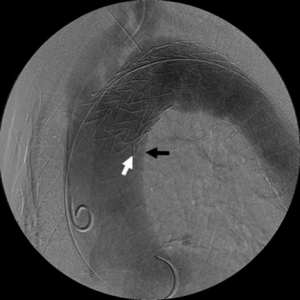
Effigy 9 Bird-nib configuration of TEVAR endograft. In that location is imperfect apposition at proximal end of the thoracic endograft to the lesser curve of the thoracic aortic arch. This lack of apposition results in a wedge-shaped gap between the undersurface of the endograft (white arrow) and the aortic wall (black arrow). The bird-nib configuration is significantly correlated with the risk of type IA endoleak germination, and it is a potential take chances factor for proximal endograft collapse or infolding. TEVAR, thoracic endovascular aneurysm repair.
The bulk of patients undergo endovascular re-intervention with the repair achieved past relining the complanate endograft (87). Relining is the preferred mode of re-intervention and provides for a more definitive solution. Available data shows that dilation of the complanate endograft without stent placement is associated with early recurrence of the collapse (xc,91). A minor percentage of patients may require surgical intervention for repair by endograft removal and open up aortic repair or past axillo-bifemoral bypass.
Systemic complications
Ischemia
Ischemic complications following EVAR have been reported in approximately nine% of cases, an incidence that is higher than is seen following open surgical repair (92). Ischemia may be caused by arterial thrombosis, embolism, arterial dissection or arterial obstruction occurring as a result of endograft malpositioning. Organs and vascular territories that may be affected by ischemia post-obit EVAR of the abdominal aorta include the kidneys, bowel, pelvic organs/muscles and the lower extremities. Spinal cord ischemia is more commonly associated with TEVAR (93). A number of cases of left upper limb ischemia, left upper extremity claudication and subclavian steal syndrome have also been reported following TEVAR (94,95).
Lower limb ischemia is among the nigh common forms of ischemia seen post-obit EVAR of the abdominal aorta with the majority occurring every bit a result of endograft limb occlusion (92), the management of which is addressed in the previous department. Limb ischemia can occur following TEVAR in the setting of inadvertent coverage of the left subclavian artery by the endograft (94-96). This is usually an infrequent complexity and is rarely symptomatic (97). More oftentimes, based upon the individual patient anatomy, left subclavian artery coverage is a planned component of the process, for which carotid-subclavian bypass or transposition is performed prior to TEVAR.
Postprocedural renal ischemia may event from arterial thrombosis embolus or autopsy, may be due to inadvertent intraprocedural coverage of the origin(s) of the renal arteries by the endograft or can result from endograft migration (98,99). A brusk aortic cervix carries an increased run a risk of inadvertent coverage of the renal arteries by the endograft. If the kidneys are not visualized on completion arteriography, stenting of the involved renal artery(ies) may be attempted. If renal function continues to deteriorate, surgical featherbed may be needed in guild to revascularize the involved kidney.
Intestinal ischemia may occur post-obit EVAR and, when present, most normally involves the colon, where it is reported to occur in i–3% of patients (100,101). Colonic ischemia is thought to result from endograft coverage of the inferior mesenteric artery origin, a miracle that occurs in all cases of EVAR of the intestinal aorta. If at that place are poorly developed mesenteric collateral arcades, left colonic ischemia may ensue. Minor bowel or correct colonic ischemia in the distribution of the superior mesenteric avenue (SMA) is much less common and may be secondary to thromboembolism from catheter and/or guidewire manipulation, specially in a long procedure or by inadvertent coverage of the SMA origin by the endograft. Bowel ischemia is far less ordinarily seen with TEVAR and has been reported when there was inadvertent coverage of the celiac artery by the distal aspect of the endograft; these may often exist less symptomatic if there is pregnant mesenteric collateralization. If there is inadvertent coverage of both the celiac trunk and SMA by the endograft, patients will likely nowadays with ischemic colitis. Patients with ischemic colitis secondary to endovascular repair typically present with abdominal hurting and encarmine diarrhea less than 30 days mail service process. A history of prior embolization of 1 or both internal iliac arteries significantly increases the chance of this complexity (101).
Pelvic ischemia has likewise been reported following EVAR of the abdominal aorta in the setting of internal iliac artery embolization. Intentional embolization of one or both internal iliac arteries has been used in patients with complex iliac arterial beefcake so equally to allow extension of endograft limbs into the external iliac arteries or to exclude internal iliac avenue aneurysms. Patient symptoms post-obit internal iliac artery embolization include buttock claudication, rectal ischemia, erectile dysfunction and skin malperfusion and necrosis. Buttock claudication has been reported in 31–35% of cases and erectile dysfunction in 17–24% of patients. Symptoms tend to improve with fourth dimension with no intervention needed. However, there is a college risk of symptoms persisting in cases in which there has been bilateral internal iliac artery embolization. Intraoperative strategies to preserve perfusion of the internal iliac arterial territories in social club to prevent these complications include investigational iliac branched devices (non currently approved past the FDA), surgical revascularization of the internal iliac artery, operator modification of currently existing endografts, and other techniques such as placement of parallel endografts.
Spinal string ischemia occurs very rarely in association with EVAR of the abdominal aorta, with approximately 14 cases reported to date (92,102,103). Unfortunately, however, the incidence of spinal ischemia is much higher with TEVAR where information technology is estimated to occur in up to 12% of cases (93). Symptoms of spinal string ischemia typically develop within 12 hours following repair and may lead to paraplegia (72). Risk factors include the extent of aortic coverage by the device, perioperative hypotension, long procedural durations, coverage of the left subclavian avenue, previous open infrarenal aortic repair and renal insufficiency (16,104). Spinal drainage tin be used in cases in which there is planned extensive coverage of the thoracic aorta to reduce the risk for spinal cord ischemia.
Cerebrovascular events
Embolic strokes accept been reported to occur in four% to 8% of the cases following TEVAR, an incidence charge per unit that is comparable to open surgery (16,29,105). The relatively high risk is a issue of the proximity of the proximal seal zone of the endovascular graft to the origins of the vertebral and carotid arteries. Adventure factors for strokes complicating TEVAR include the presence of mobile atheromata in the aortic arch, a history of prior strokes, and the need for proximal graft deployment (105). Middle cerebral apportionment strokes are most mutual, although posterior circulation strokes have been reported as a effect of embolization of debris through the vertebral arteries.
Postimplantation syndrome
Postimplantation syndrome may occur after EVAR and has a reported incidence ranging betwixt 13–60% (106,107). It is thought to represent an inflammatory immune-mediated response, with the release of inflammatory cytokines that occurs as a result of endothelial activation through a reaction to the endograft material (107). Symptoms are flu-like in nature and manifest clinically equally a systemic inflammatory response that is characterized by fever, leukocytosis, and elevated inflammatory markers, including C-reactive protein (CRP), tumor necrosis factor (TNF)-blastoff and interleukin (IL)-6 levels (92-94). Pleural effusions may occur with postimplantation syndrome and are seen in 37–73% of cases afterward TEVAR (108). Treatment consists of surveillance and aspirin administration to reduce inflammation, with no antibiotics indicated.
Open surgical conversion
Open surgical conversion involves surgical modification of an existing endovascular graft. Open conversion rates range from 0.six% to 4.5%. Surgical intervention is reserved to select cases where repair past endovascular means is not possible (sixteen,109,110). Open surgical conversion is typically needed in select cases of symptomatic type V endoleaks, or in cases involving extensive endograft migration. Aneurysm rupture typically requires removal of the endograft and repair with constructed grafts or homografts. Open up surgical repair is unremarkably of last resort peculiarly with many patients treated with EVAR not beingness good surgical candidates.
Conclusions
EVAR is increasingly being used for the handling for thoracic and AAAs and certain other aortic pathologies. It is minimally-invasive, is associated with decreased perioperative morbidity and conveys a short-term survival advantage when compared to open surgical repair. One of the disadvantages of EVAR is the relatively high incidence of post-procedural complications that thus necessitates lifelong imaging surveillance of patients. CT is the preferred method for post-procedural imaging surveillance. A number of patients require secondary re-interventions to address post-procedural endograft-related complications. Most re-interventions are pursued using an endovascular arroyo. With the increasing number of endovascular repair procedures performed, information technology is important for clinicians to gain familiarity with common complications and treatment strategies following this procedure.
Acknowledgements
None.
Conflicts of Interest: The authors have no conflicts of involvement to declare.
References
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006;43:446-51; word 451-2. [Crossref] [PubMed]
- Chaikof EL, Brewster DC, Dalman RL, et al. The care of patients with an abdominal aortic aneurysm: The Social club for Vascular Surgery practice guidelines. J Vasc Surg 2009;50:S2-49. [Crossref] [PubMed]
- Matsumura JS, Brewster DC, Makaroun MS, et al. A multicenter controlled clinical trial of open versus endovascular handling of intestinal aortic aneurysm. J Vasc Surg 2003;37:262-71. [Crossref] [PubMed]
- Stone DH, Brewster DC, Kwolek CJ, et al. Stent-graft versus open up-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg 2006;44:1188-97. [Crossref] [PubMed]
- Najibi S, Terramani TT, Weiss VJ, et al. Endoluminal versus open treatment of descending thoracic aortic aneurysms. J Vasc Surg 2002;36:732-seven. [Crossref] [PubMed]
- Bavaria JE, Appoo JJ, Makaroun MS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in depression-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg 2007;133:369-77. [Crossref] [PubMed]
- Makaroun MS, Dillavou ED, Kee ST, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 2005;41:1-9. [Crossref] [PubMed]
- Carpenter JP, Anderson WN, Brewster DC, et al. Multicenter pivotal trial results of the Lifepath Arrangement for endovascular aortic aneurysm repair. J Vasc Surg 2004;39:34-43. [Crossref] [PubMed]
- Makaroun MS, Dillavou ED, Wheatley GH, et al. Five-year results of endovascular treatment with the Gore TAG device compared with open up repair of thoracic aortic aneurysms. J Vasc Surg 2008;47:912-eight. [Crossref] [PubMed]
- Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of intestinal aortic aneurysm sac enlargement later endovascular repair. Circulation 2022;123:2848-55. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2022 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic affliction: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association forThoracic Surgery, American College of Radiology, American Stroke Association, Lodge of Cardiovascular Anesthesiologists, Lodge for Cardiovascular Angiography and Interventions, Gild of Interventional Radiology, Gild of Thoracic Surgeons, and Lodge for Vascular Medicine. Catheter Cardiovasc Interv 2022;76:E43-86. [Crossref] [PubMed]
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for Aortic Dilatation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the American College of Cardiology/American Heart Association Chore Force on Clinical Practice Guidelines. J Am Coll Cardiol 2022;67:724-31. [Crossref] [PubMed]
- Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 2006;81:169-77. [Crossref] [PubMed]
- Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 2006;81:169-77. [Crossref] [PubMed]
- Lederle FA, Kane RL, MacDonald R, et al. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med 2007;146:735-41. [Crossref] [PubMed]
- Teufelsbauer H, Prusa AM, Wolff G, et al. Endovascular stent grafting versus open surgical functioning in patients with infrarenal aortic aneurysms: a propensity score-adjusted analysis. Circulation 2002;106:782-seven. [Crossref] [PubMed]
- Greenberg RK, Lu Q, Roselli EE, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation 2008;118:808-17. [Crossref] [PubMed]
- Giles KA, Pomposelli F, Hamdan A, et al. Subtract in full aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009;49:543-50; discussion 550-1. [Crossref] [PubMed]
- Giles KA, Schermerhorn ML, O'Malley AJ, et al. Risk prediction for perioperative mortality of endovascular vs open up repair of intestinal aortic aneurysms using the Medicare population. J Vasc Surg 2009;50:256-62. [Crossref] [PubMed]
- Sadat U, Boyle JR, Walsh SR, et al. Endovascular vs open up repair of acute intestinal aortic aneurysms--a systematic review and meta-analysis. J Vasc Surg 2008;48:227-36. [Crossref] [PubMed]
- Walsh SR, Tang TY, Sadat U, et al. Endovascular stenting versus open surgery for thoracic aortic disease: systematic review and meta-analysis of perioperative results. J Vasc Surg 2008;47:1094-8. [Crossref] [PubMed]
- Schermerhorn ML, O'Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008;358:464-74. [Crossref] [PubMed]
- Walker TG, Kalva SP, Yeddula K, et al. Clinical practice guidelines for endovascular intestinal aortic aneurysm repair: written by the Standards of Practice Commission for the Society of Interventional Radiology and endorsed by the Cardiovascular and Interventional Radiological Guild of Europe and the Canadian Interventional Radiology Association. J Vasc Interv Radiol 2022;21:1632-55. [Crossref] [PubMed]
- Prinssen M, Verhoeven EL, Buth J, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. Due north Engl J Med 2004;351:1607-xviii. [Crossref] [PubMed]
- Nordon IM, Karthikesalingam A, Hinchliffe RJ, et al. Secondary interventions post-obit endovascular aneurysm repair (EVAR) and the enduring value of graft surveillance. Eur J Vasc Endovasc Surg 2022;39:547-54. [Crossref] [PubMed]
- Drury D, Michaels JA, Jones 50, et al. Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal intestinal aortic aneurysm. Br J Surg 2005;92:937-46. [Crossref] [PubMed]
- U.k. EVAR Trial Investigators, Greenhalgh RM, Brown LC, et al. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med 2022;362:1872-80. [Crossref] [PubMed]
- Brown LC, Greenhalgh RM, Powell JT, et al. Utilize of baseline factors to predict complications and reinterventions after endovascular repair of abdominal aortic aneurysm. Br J Surg 2022;97:1207-17. [Crossref] [PubMed]
- Karthikesalingam A, Holt PJ, Hinchliffe RJ, et al. Risk of reintervention after endovascular aortic aneurysm repair. Br J Surg 2022;97:657-63. [Crossref] [PubMed]
- Dake MD, Miller DC, Mitchell RS, et al. The "first generation" of endovascular stent-grafts for patients with aneurysms of the descending thoracic aorta. J Thorac Cardiovasc Surg 1998;116:689-703; discussion 703-4. [Crossref] [PubMed]
- Matsumura JS, Cambria RP, Dake Dr., et al. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg 2008;47:247-57; give-and-take 257. [Crossref] [PubMed]
- Scali ST, Beck AW, Butler K, et al. Pathology-specific secondary aortic interventions afterwards thoracic endovascular aortic repair. J Vasc Surg 2022;59:599-607. [Crossref] [PubMed]
- Szeto WY, Desai ND, Moeller P, et al. Reintervention for endograft failures afterwards thoracic endovascular aortic repair. J Thorac Cardiovasc Surg 2022;145:S165-seventy. [Crossref] [PubMed]
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Do Guidelines for the management of patients with peripheral arterial affliction (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Guild for Vascular Surgery, Social club for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Gild of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Middle, Lung, and Blood Establish; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Apportionment 2006;113:e463-654. [Crossref] [PubMed]
- Shah A, Stavropoulos SW. Imaging Surveillance following Endovascular Aneurysm Repair. Semin Intervent Radiol 2009;26:x-half-dozen. [Crossref] [PubMed]
- Murphy M, Hodgson R, Harris PL, et al. Apparently radiographic surveillance of abdominal aortic stent-grafts: the Liverpool/Perth protocol. J Endovasc Ther 2003;10:911-2. [Crossref] [PubMed]
- Armerding Dr., Rubin GD, Beaulieu CF, et al. Aortic aneurysmal illness: assessment of stent-graft treatment-CT versus conventional angiography. Radiology 2000;215:138-46. [Crossref] [PubMed]
- Ducasse E, Calisti A, Speziale F, et al. Aortoiliac stent graft infection: current issues and management. Ann Vasc Surg 2004;18:521-6. [Crossref] [PubMed]
- Picel Air-conditioning, Kansal N. Essentials of endovascular abdominal aortic aneurysm repair imaging: postprocedure surveillance and complications. AJR Am J Roentgenol 2022;203:W358-72. [PubMed]
- Stolzmann P, Frauenfelder T, Pfammatter T, et al. Endoleaks after endovascular abdominal aortic aneurysm repair: detection with dual-free energy dual-source CT. Radiology 2008;249:682-91. [Crossref] [PubMed]
- Hansen NJ, Kaza RK, Maturen KE, et al. Evaluation of depression-dose CT angiography with model-based iterative reconstruction after endovascular aneurysm repair of a thoracic or abdominal aortic aneurysm. AJR Am J Roentgenol 2022;202:648-55. [Crossref] [PubMed]
- Schindera ST, Graca P, Patak MA, et al. Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: assessment of image quality and radiation dose. Invest Radiol 2009;44:650-five. [Crossref] [PubMed]
- Haulon S, Lions C, McFadden EP, et al. Prospective evaluation of magnetic resonance imaging later endovascular treatment of infrarenal aortic aneurysms. Eur J Vasc Endovasc Surg 2001;22:62-nine. [Crossref] [PubMed]
- Habets J, Zandvoort HJ, Reitsma JB, et al. Magnetic resonance imaging is more sensitive than computed tomography angiography for the detection of endoleaks after endovascular abdominal aortic aneurysm repair: a systematic review. Eur J Vasc Endovasc Surg 2022;45:340-fifty. [Crossref] [PubMed]
- Resta EC, Secchi F, Giardino A, et al. Not-contrast MR imaging for detecting endoleak later abdominal endovascular aortic repair. Int J Cardiovasc Imaging 2022;29:229-35. [Crossref] [PubMed]
- Cohen EI, Weinreb DB, Siegelbaum RH, et al. Time-resolved MR angiography for the classification of endoleaks afterward endovascular aneurysm repair. J Magn Reson Imaging 2008;27:500-iii. [Crossref] [PubMed]
- Olchowy C, Cebulski One thousand, Łasecki Grand, et al. The presence of the gadolinium-based dissimilarity agent depositions in the encephalon and symptoms of gadolinium neurotoxicity - A systematic review. PLoS One 2022;12:e0171704. [Crossref] [PubMed]
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial Gadolinium Deposition after Dissimilarity-enhanced MR Imaging. Radiology 2022;275:772-82. [Crossref] [PubMed]
- Millen A, Canavati R, Harrison G, et al. Defining a role for dissimilarity-enhanced ultrasound in endovascular aneurysm repair surveillance. J Vasc Surg 2022;58:18-23. [Crossref] [PubMed]
- Abraha I, Luchetta One thousand, De Florio R, et al. Ultrasonography versus computed tomography scan for endoleak detection later endoluminal abdominal aortic aneurysm repair. Cochrane Database Syst Rev 2022. [Crossref]
- Raman KG, Missig-Carroll N, Richardson T, et al. Colour-flow duplex ultrasound browse versus computed tomographic browse in the surveillance of endovascular aneurysm repair. J Vasc Surg 2003;38:645-51. [Crossref] [PubMed]
- Ashoke R, Brown LC, Rodway A, et al. Colour duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. J Endovasc Ther 2005;12:297-305. [Crossref] [PubMed]
- Mark AS, McCarthy SM, Moss AA, et al. Detection of abdominal aortic graft infection: comparison of CT and in-labeled white blood jail cell scans. AJR Am J Roentgenol 1985;144:315-8. [Crossref] [PubMed]
- Johnson KK, Russ PD, Bair JH, et al. Diagnosis of synthetic vascular graft infection: comparison of CT and gallium scans. AJR Am J Roentgenol 1990;154:405-9. [Crossref] [PubMed]
- Fiorani P, Speziale F, Rizzo L, et al. Detection of aortic graft infection with leukocytes labeled with technetium 99m-hexametazime. J Vasc Surg 1993;17:87-95; discussion 95-6. [Crossref] [PubMed]
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for direction. Radiographics 2000;20:977-93. [Crossref] [PubMed]
- Hovsepian DM, Siegel BA, Kimbiris 1000, et al. Tc-99m sulfur colloid scintigraphy for detecting perigraft flow following endovascular aortic aneurysm repair: A feasibility report. Cardiovasc Intervent Radiol 1999;22:447-51. [Crossref] [PubMed]
- Stavropoulos SW, Itkin M, Lakhani P, et al. Detection of endoleaks after endovascular aneurysm repair with utilize of technetium-99m sulfur colloid and (99m)Tc-labeled cherry blood jail cell scans. J Vasc Interv Radiol 2006;17:1739-43. [Crossref] [PubMed]
- Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg 2022;41 Suppl 1:S1-58. [Crossref] [PubMed]
- Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002;35:1048-60. [Crossref] [PubMed]
- O'Connor Due south, Andrew P, Batt M, et al. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg 2006;44:38-45. [Crossref] [PubMed]
- Cernohorsky P, Reijnen MM, Tielliu IF, et al. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2022;54:327-33. [Crossref] [PubMed]
- Liaw JV, Clark M, Gibbs R, et al. Update: Complications and direction of infrarenal EVAR. Eur J Radiol 2009;71:541-51. [Crossref] [PubMed]
- Parmer SS, Carpenter JP, Stavropoulos SW, et al. Endoleaks after endovascular repair of thoracic aortic aneurysms. J Vasc Surg 2006;44:447-52. [Crossref] [PubMed]
- Faries PL, Cadot H, Agarwal G, et al. Management of endoleak afterwards endovascular aneurysm repair: cuffs, coils, and conversion. J Vasc Surg 2003;37:1155-61. [Crossref] [PubMed]
- Tzortzis Eastward, Hinchliffe RJ, Hopkinson BR. Adjunctive procedures for the treatment of proximal blazon I endoleak: the office of peri-aortic ligatures and Palmaz stenting. J Endovasc Ther 2003;x:233-nine. [Crossref] [PubMed]
- Kirby L, Goodwin J. Treatment of a master type IA endoleak with a liquid embolic organisation under weather condition of aortic apoplexy. J Vasc Surg 2003;37:456-60. [Crossref] [PubMed]
- Deaton DH, Mehta 1000, Kasirajan Grand, et al. The stage I multicenter trial (STAPLE-1) of the Aptus endovascular repair system: results at 6 months and one yr. J Vasc Surg 2009;49:851-seven; discussion 857-eight. [Crossref] [PubMed]
- Bail DH, Walker T, Giehl J. Vascular endostapling systems for vascular endografts (T)EVAR--systematic review--current state. Vasc Endovascular Surg 2022;47:261-6. [Crossref] [PubMed]
- Bonvini R, Alerci Thou, Antonucci F, et al. Preoperative embolization of collateral side branches: a valid ways to reduce type Ii endoleaks after endovascular AAA repair. J Endovasc Ther 2003;10:227-32. [Crossref] [PubMed]
- Stavropoulos SW, Carpenter JP, Fairman RM, et al. Junior vena cava traversal for translumbar endoleak embolization after endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol 2003;14:1191-four. [Crossref] [PubMed]
- Alvarez-Tostado JA, Moise MA, Bena JF, et al. The brachial artery: a critical access for endovascular procedures. J Vasc Surg 2009;49:378-85; discussion 385. [Crossref] [PubMed]
- Mansueto M, Cenzi D, D'Onofrio M, et al. Treatment of type Ii endoleaks after endovascular repair of intestinal aortic aneurysms: transcaval arroyo. Cardiovasc Intervent Radiol 2005;28:641-5. [Crossref] [PubMed]
- Ellis PK, Kennedy PT, Collins AJ, et al. The employ of straight thrombin injection to treat a type II endoleak following endovascular repair of abdominal aortic aneurysm. Cardiovasc Intervent Radiol 2003;26:482-four. [Crossref] [PubMed]
- Wisselink W, Cuesta MA, Berends FJ, et al. Retroperitoneal endoscopic ligation of lumbar and inferior mesenteric arteries as a treatment of persistent endoleak after endoluminal aortic aneurysm repair. J Vasc Surg 2000;31:1240-iv. [Crossref] [PubMed]
- Laheij RJ, Buth J, Harris PL, et al. Demand for secondary interventions after endovascular repair of abdominal aortic aneurysms. Intermediate-term follow-up results of a European collaborative registry (EUROSTAR). Br J Surg 2000;87:1666-73. [Crossref] [PubMed]
- Tonnessen BH, Sternbergh WC tertiary, Money SR. Mid- and long-term device migration subsequently endovascular abdominal aortic aneurysm repair: a comparison of AneuRx and Zenith endografts. J Vasc Surg 2005;42:392-400; give-and-take 400-1. [Crossref] [PubMed]
- Deaton DH. Improving proximal fixation and seal with the HeliFx Aortic EndoAnchor. Semin Vasc Surg 2022;25:187-92. [Crossref] [PubMed]
- Ohki T, Ouriel Thou, Silveira PG, et al. Initial results of wireless pressure level sensing forendovascular aneurysm repair: the Noon Trial--Acute Pressure Measurement to Ostend Aneurysm Sac EXclusion. J Vasc Surg 2007;45:236-42. [Crossref] [PubMed]
- Murphy EH, Szeto WY, Herdrich BJ, et al. The management of endograft infections following endovascular thoracic and abdominal aneurysm repair. J Vasc Surg 2022;58:1179-85. [Crossref] [PubMed]
- Capoccia L, Speziale F, Menna D, et al. Preliminary Results from a National Research of Infection in Abdominal Aortic Endovascular Repair (Registry of Infection in EVAR--R.I.EVAR). Ann Vasc Surg 2022;thirty:198-204. [Crossref] [PubMed]
- Smeds MR, Duncan AA, Harlander-Locke MP, et al. Treatment and outcomes of aortic endograft infection. J Vasc Surg 2022;63:332-xl. [Crossref] [PubMed]
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection afterwards aortic aneurysm repair. J Vasc Surg 2008;47:264-9. [Crossref] [PubMed]
- Sharif MA, Lee B, Lau LL, et al. Prosthetic stent graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2007;46:442-8. [Crossref] [PubMed]
- EVAR trial participants. Endovascular aneurysm repair versus open up repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179-86. [Crossref] [PubMed]
- Fransen GA, Desgranges P, Laheij RJ, et al. Frequency, predictive factors, and consequences of stent-graft kink following endovascular AAA repair. J Endovasc Ther 2003;ten:913-8. [Crossref] [PubMed]
- Jonker FH, Schlosser FJ, Geirsson A, et al. Endograft plummet afterward thoracic endovascular aortic repair. J Endovasc Ther 2022;17:725-34. [Crossref] [PubMed]
- Tadros RO, Lipsitz EC, Chaer RA, et al. A multicenter experience of the management of complanate thoracic endografts. J Vasc Surg 2022;53:1217-22. [Crossref] [PubMed]
- Kasirajan K, Dake Md, Lumsden A, et al. Incidence and outcomes afterwards infolding or collapse of thoracic stent grafts. J Vasc Surg 2022;55:652-8; word 658. [Crossref] [PubMed]
- Sze DY, Mitchell RS, Miller DC, et al. Infolding and collapse of thoracic endoprostheses: manifestations and treatment options. J Thorac Cardiovasc Surg 2009;138:324-33. [Crossref] [PubMed]
- Bandorski D, Brück M, Günther HU, et al. Endograft plummet later endovascular treatment for thoracic aortic disease. Cardiovasc Intervent Radiol 2022;33:492-seven. [Crossref] [PubMed]
- Maldonado TS, Rockman CB, Riles East, et al. Ischemic complications after endovascular intestinal aortic aneurysm repair. J Vasc Surg 2004;xl:703-9; discussion 709-10. [Crossref] [PubMed]
- Setacci F, Sirignano P, De Donato G, et al. Endovascular thoracic aortic repair and risk of spinal string ischemia: the part of previous or concomitant handling for aortic aneurysm. J Cardiovasc Surg (Torino) 2022;51:169-76. [PubMed]
- Si Y, Fu W, Liu Z, et al. Coverage of the left subclavian artery without revascularization during thoracic endovascular repair is feasible: a prospective study. Ann Vasc Surg 2022;28:850-9. [Crossref] [PubMed]
- Klocker J, Koell A, Erlmeier 1000, et al. Ischemia and functional status of the left arm and quality of life after left subclavian artery coverage during stent grafting of thoracic aortic diseases. J Vasc Surg 2022;sixty:64-9. [Crossref] [PubMed]
- Riesenman PJ, Farber MA, Mendes RR, et al. Coverage of the left subclavian artery during thoracic endovascular aortic repair. J Vasc Surg 2007;45:ninety-four; word 94-5. [Crossref] [PubMed]
- Maldonado TS, Dexter D, Rockman CB, et al. Left subclavian artery coverage during thoracic endovascular aortic aneurysm repair does non mandate revascularization. J Vasc Surg 2022;57:116-24. [Crossref] [PubMed]
- Chang CK, Chuter TA, Niemann CU, et al. Systemic inflammation, coagulopathy,and acute renal insufficiency following endovascular thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:1140-vi. [Crossref] [PubMed]
- Dark-brown LC, Brown EA, Greenhalgh RM, et al. Renal office and abdominal aortic aneurysm (AAA): the touch on of different management strategies on long-term renal office in the Great britain EndoVascular Aneurysm Repair (EVAR) Trials. Ann Surg 2022;251:966-75. [Crossref] [PubMed]
- Becquemin JP, Majewski Yard, Fermani N, et al. Colon ischemia following abdominal aortic aneurysm repair in the era of endovascular intestinal aortic repair. J Vasc Surg 2008;47:258-63; discussion 263. [Crossref] [PubMed]
- Miller A, Marotta K, Scordi-Bello I, et al. Ischemic colitis after endovascular aortoiliac aneurysm repair: a 10-yr retrospective study. Arch Surg 2009;144:900-3. [Crossref] [PubMed]
- Angiletta D, Marinazzo D, Guido G, et al. Spinal cord, bowel, and buttock ischemia after endovascular aneurysm repair. Ann Vasc Surg 2022;25:980.e15-9. [Crossref] [PubMed]
- Freyrie A, Testi Thou, Gargiulo Chiliad, et al. Spinal cord ischemia afterwards endovascular handling of infrarenal aortic aneurysm. Case report and literature review. J Cardiovasc Surg (Torino) 2022;52:731-iv. [PubMed]
- Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and run a risk factors. a report from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg 2007;46:1103-10; word 1110-1. [Crossref] [PubMed]
- Gutsche JT, Cheung AT, McGarvey ML, et al. Chance factors for perioperative stroke after thoracic endovascular aortic repair. Ann Thorac Surg 2007;84:1195-200; discussion 1200. [Crossref] [PubMed]
- Moulakakis KG, Dalainas I, Mylonas South, et al. Conversion to open repair after endografting for intestinal aortic aneurysm: a review of causes, incidence, results, and surgical techniques of reconstruction. J Endovasc Ther 2022;17:694-702. [Crossref] [PubMed]
- Gabriel EA, Locali RF, Romano CC, et al. Analysis of the inflammatory response in endovascular treatment of aortic aneurysms. Eur J Cardiothorac Surg 2007;31:406-12. [Crossref] [PubMed]
- Eggebrecht H, Mehta RH, Metozounve H, et al. Clinical implications of systemic inflammatory response syndrome following thoracic aortic stent-graft placement. J Endovasc Ther 2008;xv:135-43. [Crossref] [PubMed]
- Lyden SP, McNamara JM, Sternbach Y, et al. Technical considerations for belatedly removal of aortic endografts. J Vasc Surg 2002;36:674-8. [Crossref] [PubMed]
- Conner MS 3rd, Sternbergh WC 3rd, Carter G, et al. Secondary procedures after endovascular aortic aneurysm repair. J Vasc Surg 2002;36:992-6. [Crossref] [PubMed]
Cite this article as: Daye D, Walker TG. Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management. Cardiovasc Diagn Ther 2022;8(Suppl 1):S138-S156. doi: x.21037/cdt.2017.09.17
Source: https://cdt.amegroups.com/article/view/16911/19122
Posted by: grimeswint1956.blogspot.com


0 Response to "Most Common Immediate Complication Following Surgical Repair Of An Abdominal Aortic Aneurysm?"
Post a Comment